Brain Estrogen & Neurotransmitter Signaling
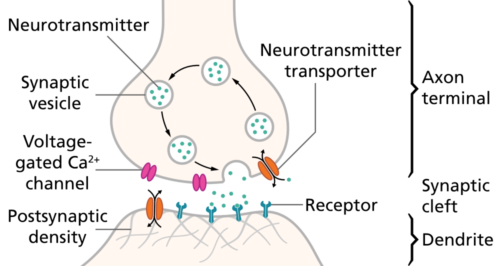
Neurons in the brain signal to each other with the release of a chemical called a neurotransmitter. Until the last few years, it was believed that neurotransmitter molecules were all amino acid derivatives or chains of amino acids, peptides or proteins.
Steroids like estrogen, cholesterol derivatives were never thought useful for this purpose, because they rapidly distribute themselves in tissue, passing easily through cell membranes. A timed, discreet binding to a postsynaptic ion channel receptor seemed impossible.
Yet, since the 1970s it has been known that the application of estradiol, a cholesterol derivative, to local areas of the brain caused a rapid change in the firing rate of specific neurons. The time course of the response could not be explained by estrogen binding to its classical receptor in the nucleus of neurons, a much slower process requiring hours.
What may estradiol’s ability to alter firing rate and synaptic transmission of brain neurons mean to onset of Alzheimer’s disease? Estradiol is the primary female hormone, and it is known that being female increases the risk of developing Alzheimer’s disease and of displaying severe memory loss.
Two thirds of Alzheimer’s disease cases are women. Women express a broader range of symptoms than men, and memory loss is greater in women. Some protective effect of estrogen replacement at the beginning of menopause is observed. An answer to the puzzle of how estrogen can protect against synapse loss observed in Alzheimer’s disease is easier to imagine if estradiol actually is a neurotransmitter.
Brain Steroids
It is difficult to imagine lipid-soluble brain steroids as as neurotransmitter. Steroids like estradiol cannot be packaged in vesicles for release upon the arrival of action potentials at the presynaptic compartment. Estradiol diffuses rapidly through cell membranes and distributes evenly through the brain as it does in other tissues.
Steroids are made from cholesterol in the ovary, testes, adrenal glands and brain. Steroid hormones from peripheral glands travel in blood and through interstitial fluid to find their target cells. Areas of the brain respond to ovarian estradiol in a well-studied pattern that supports female reproduction. But overlaid on the cyclic presence of reproductive hormone is brain’s own regional synthesis of estradiol.
The name hormone originates from a Greek word that means to ‘set in motion’. If a cell has a detector for the presence of a hormone, then that hormone is capable of setting in motion the cell’s molecular pathways. Initiation of a hormone response depends upon the target cell’s set of proteins. Each hormone binds to a unique protein designated its hormone receptor.
Since the 1970s, ovarian estradiol has been known to bind to a soluble hormone receptor in the nucleus of target cells. When bound with estrogen, this receptor forms a complex with other nuclear proteins. The estradiol complex activates specific regions of the DNA to produce message for synthesis of new protein, setting molecular pathways in motion.
The time from estradiol binding to its nuclear receptor to the appearance of new cellular proteins takes hours, sometimes as long as 24 hours. This is too long to explain the observed response of brain regions to application of exogenous estradiol within a few seconds.
Multiple Types of Estrogen Receptors
Steroid hormones influence cellular pathways by binding to receptor proteins located within their target cells. It took decades of research to discover the many types of steroid hormone receptors used by the body.
Missing pieces to the puzzling time course of estradiol activity in the brain emerged slowly over about 50 years. A major finding since the 1970s is that there are multiple types of estradiol receptor. In addition to the classical nuclear estrogen receptor first describe in the 1960s, a second estrogen receptor isoform coded by a separate gene was reported in 1996. These estrogen receptor isoforms are now known as ERα and ERβ.

Structural Organization of Nuclear Receptors. 3D structures are of the estrogen receptor (ERα). Released into the public domain by Boghog2/English Wikipedia.
Both ERα and ERβ are present in estrogen target organs throughout the body including certain areas of the human brain. Both can act as classical nuclear transcription factors over a time course of hours. ERα is generally more involved in the central nervous system’s early development and the brain’s role in reproductive behavior. ERβ it thought to have a greater role in cognitive processes.
There are, however, estrogen effects that are not blocked by blocking DNA transcription suggesting the presence of additional forms of estradiol receptor.
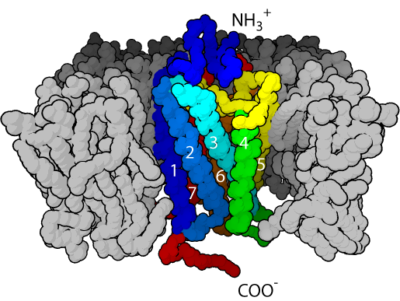
G-Protein Coupled Receptor. This work has been released into the public domain by its author, Bensaccount at English Wikipedia.
As early as 1967 application of estradiol to the rat uterus showed an increase in cellular cyclic AMP levels by 15 seconds. Beginning in 1999 and through the early years of the 21st century an increasing quantity of evidence confirmed the presence of membrane embedded proteins performing as estradiol receptors. Estradiol membrane bound receptors (mER), like other membrane receptors, are characterized by their rapid and transient activation of numerous cellular signaling pathways.
Membrane receptors for estrogen include ERα and ERβ, and G-Protein Coupled Receptors (GPCRs). GPCRs are a group of receptors with a structure that passes seven times through a cellular membrane. They are often activated by amines derivative molecules. Brain mER is found in the membrane of dendrites, of dendritic spines, of axons, of postsynaptic densities and in the active zone of some presynaptic compartments.
Other cellular compartments displaying mER include the Golgi, endoplasmic reticulum, plasma membrane clatherin-coated pits and early endosomes. In brain mER are found in the frontal cortex, the basal forebrain, thalamus, hippocampus, caudate nucleus and putamen. They are also found in endothelial cells lining blood vessels.
The brain contains GPCR receptors near synapses that respond to neurotransmitter molecules, but not as part of neuron synaptic transmission. Rather, those GPCRs set in motion cellular pathways that may enhance or reduce the effectiveness of the synapse’s ion channel receptors.
Estradiol Synthesis & Secretion
Estradiol synthesis in the brain uses the same pathway as its synthesis in the ovary. Clearly an array of non-nuclear estradiol receptors exists in the brain and elsewhere in the body. But there is still the problem of the lipid solubility of estradiol. What is to keep estradiol from diffusing indiscriminately throughout the brain? The answer rests with how estradiol is synthesized at brain synapses. As you can see from the figure of the steroid hormone synthetic pathways, many steroids are precursor substrate for others. Notice the immediate precursors for estradiol is the male hormone testosterone.
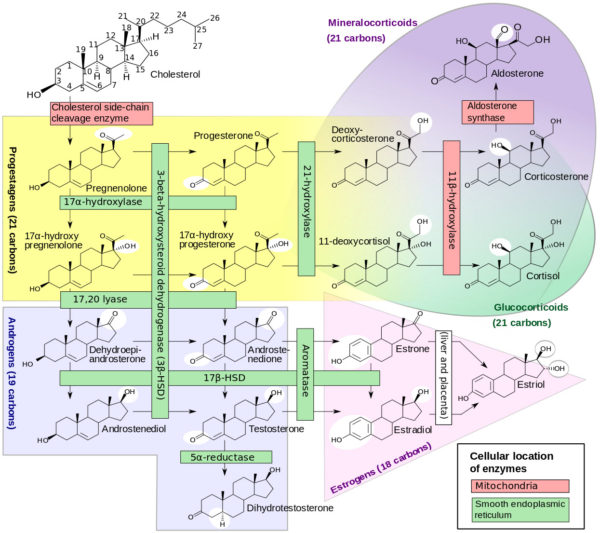
Formation of steroid hormones from cholesterol, David Richfield and Mikael Häggström/Wikimedia Commons
The enzyme that converts testosterone to estradiol is named aromatase. Aromatase converts the A ring of the testosterone molecule from a ketone to an aromatic ring. All of the enzymes necessary to make estradiol from brain cholesterol are be present in the human brain. However, aromatase is restricted in mammals to neurons in distinct neuronal circuits. Brain areas rich in aromatase include the hypothalamus, bed nucleus of the stria terminalis and amygdala.
In theory for estradiol to perform as a neurotransmitter, synapse aromatase activity must increase briefly in response to action potentials. That is, secretion of estradiol at the synapse must be tightly linked to its synthesis.While the precise mechanism of regulation of estradiol synthesis at neuron synapses remains unclear, studies of reproductive behavior of song birds and rodents suggest that synapse aromatase activity is timed appropriately for estradiol to serve as a neurotransmitter.
Calcium ion entering the presynaptic terminal with arriving action potentials is one potential regulator of estradiol synthesis in the synapse. Calcium is able to reduce activity of aromatase by phosphorylating it. Yet, the time required for the phosphorylation reaction, about 10 seconds, is slow compared to inactivation of other neurotransmitters by re-uptake mechanisms.
Further Reading
For information about the research supporting estradiol as a neurotransmitter and how failure of this system may impact Alzheimer’s disease, read the following references. The link with each takes you to PubMed at the National Center for Biotechnology Information in the United States where you may download these papers for free.
Rudolph LM, Cornil CA, Mittelman-Smith MA, Rainville, JR, Remage-Healey L, Sinchak K, Micevych PE (2016) Actions of Steroids: New Neurotransmitters. J Neurosci 36(45):11449-11458 PMID:27911748 click here.
Oberlander JG and Woolley CS (2016) 17β – Estradiol Acutely Potentiates Glutamatergic Synaptic Transmission in the Hippocampus through Distinct Mechanisms in Males and Females. J. Neurosci 36(9):2677-2690 PMID:26937008 click here.
Zhao L, Woody SK, Chhibber A (2015) Estrogen Receptor β in Alzheimer’s Disease: From Mechanism to Therapeutics. Aging Res Rev Nov;24(Pt B):178-90. doi: 10.1016/j.arr.2015.08.001. Epub 2015 Aug 22 PMID:26307455 click here.
Kenealy BP, Kapoor A, Guerriero KA, Keen KL, Garcia JP, Kurian JR, Ziegler TE, Terasawa E (2013) Neruoestradiol in the Hypothalamus Contributes to the Regulation of Gonadotropin Releasing Hormone Release. J Neruosci 33(49):19051-19059 PMID:26496022 click here.
Soltysik K, Czekaj P (2013) Membrane Estrogen Receptors – Is It An Alternative Way of Estrogen Action? J Physiol Pharmacol 64(2):129-142 PMID:23756388 click here.
Szego CM, Davis JS (1967) Adenosine 3′,5′-Monophosphate in Rat Uterus: Acute Elevation by Estrogen. Proc Natl Acad Sci USA 58(4):1711-1718 PMID:4295833 click here.