A Risk Factor for Alzheimer’s or a Cause of Disease ?
To figure out where brain function slowly goes awry over decades, it seems reasonable to look for clues in how it normally carries out its duties. Toward that goal I begin with today’s topic, brain cholesterol homeostasis.
Moving cholesterol around within the brain from cell to cell requires a protein named apolipoprotein. There are three normal forms of apolipoprotein used by humans, APOE2, APOE3, and APOE4. APOE4 has gained attention because only about 15% of humans use that form, but about 60% of people living with Alzheimer’s disease use that form of apolipoprotein.
Brain Cholesterol Synthesis and Distribution
What is unique about cholesterol synthesis and distribution within the human brain? Cholesterol found in the brain is made in the brain. Circulating cholesterol made in the liver does not cross the blood brain barrier at brain capillaries.
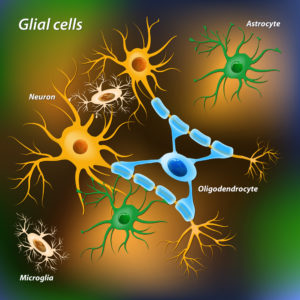
Brain cholesterol is synthesized primarily by astrocytes and oligodendrocytes from acetyl-CoA in a series of enzyme reactions on the cell’s inner membranes called endoplasmic reticulum.
About 90% of brain’s cells are not neurons. The non-neuron cells of the brain are called glia. There are three types of glial cells, astrocyte, oligodendrocytes and microglia.
In astrocytes, the major producer of brain cholesterol, acetyl-CoA is made from pyruvate in the matrix of the cell’s powerhouse, the mitochondria. Pyruvate is a product of the breakdown of the brain’s primary nutrient glucose.
Pyruvate is able to move from the cell’s cytoplasm where it is produced into mitochondria. But, acetyl CoA made in the mitochondria from pyruvate cannot leave the mitochondria to travel to the cell’s endoplasmic reticulum to make cholesterol. To solve this problem, acetyl CoA is returned to the cytoplasm by a roundabout mechanism that includes citrate. Citrate, made from acetyl CoA and another molecule, is able to leave the mitochondria and can then be reverse-metabolized back to acetyl-CoA.
Cholesterol destined for export from astrocytes is assembled into lipoprotein particles in the endoplasmic reticulum to increase its solubility in water. Apolipoprotein E (APOE) is the carrier protein for cholesterol in the brain. APOE is highly expressed in astrocytes but not in neurons. Proteins called ATP-binding cassette transporters transfer APOE lipoproteins particles across the astrocyte plasma membrane into the interstitial fluid for delivery to neurons and oligodendrocytes. About 80% of brain’s cholesterol is found in oligodendrocyte myelin that surrounds neuron axons.
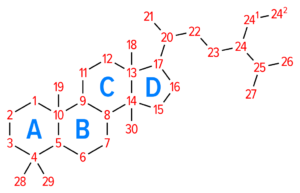
In addition to its use in oligodendrocyte myelin, cholesterol is added to all brain cell membranes and it is converted to steroid molecules. Steroids synthesized in the brain are named Neurosteroids to distinguish them from steroid hormones produced in peripheral endocrine glands. Cholesterol reserves are stored in cells as esters that form lipid droplets.
Apolipoprotein is Not Unique to Mammels
Apolipoprotein is not unique to the brain or to mammals. APOE is also used by the liver to carry cholesterol and phospholipids in the periphery. For the past 7.5 million years, there have been 3 major alleles of APOE expressed in people, APOE4, APOE3 and APOE2. The first expression of APOE, APOE4 appeared in fish about 400 million years ago.
A single amino acid change about 200,000 years ago resulted in the APOE3 allele. Then another amino acid change about 80,000 years ago created the APOE2 allele. Expression of the APOE2 and APOE3 allele appears to be associated with a survival advantage, because only 15-20% of the human population now carry even one copy of APOE4.
Neuron Uptake of Lipoprotein
Neurons depend on lipoprotein fpr uptake for their cholesterol. APOE lipoprotein, a low-density lipoprotein (LDL), is taken into neurons by large plasma membrane proteins named low-density lipoprotein receptor (LDLR) and lipoprotein-like receptor protein 1 (LRP1). Endosomes form around the lipoprotein-bound receptors and move them into the cell.
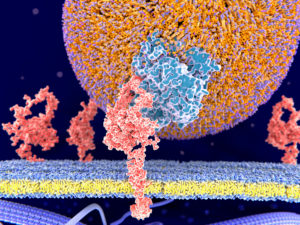
This illustration depicts an APO particle is in blue. The orange surface of the ball is composed of phospholipids with their headpieces shown in purple. Cholesterol is enclosed in the center of the large ball.
Inside brain neurons, endosomes fuse with lysosomes. Lysosomal enzymes digest the receptor-bound APOE particle releasing cholesterol. However, cholesterol still needs the help of two more transporter proteins, Nieman-Pick C1 protein (NPC1) and Nieman-Pick C2 protein (NPC2), to find its way through the endosome/lysosome membrane.
NPC2 loads the cholesterol released from the center of the particle onto NPC1. NCP1 then takes cholesterol through the membrane into the cell’s cytoplasm. From there cholesterol moves to the cell’s endoplasmic reticulum and other cell membranes by what is still and unclear mechanism.
Loss of function of NPC1 and NPC2 leads to accumulation of cholesterol in lysosomes in the periphery and in the central nervous system. Resulting clinical problems include, among others, progressive ataxia, loss of full control of body movements, and dementia.
Brain Cholesterol Homeostasis
Cholesterol plays and important role in brain homeostasis. Homeostasis is a term usually used in physiology to describe mechanisms to keep a particular variable, like pH and body temperature within a set quantitative range. In the case of cholesterol, homeostasis refers to assuring that there is neither too much nor too little of it available.
Too much or too little cholesterol in a portion of plasma membrane leads to serious functional problems.
Membranes are composed of a bilayer of phospholipids. The energy level of the phospholipids, that is their ability to move within the membrane, is influenced by their local environment and temperature. Inserting cholesterol between phospholipids lowers their energy level and makes the membrane less fluid.
Check out a nice short video about membrane dynamics produced by KHANAcademy. Click here to watch.
Cholesterol homeostasis takes co-ordination between the cell populations of the brain, because neurons rely primarily on other cells to make its much-needed cholesterol. Some surplus, or reservoir cholesterol, can be stored within cells as esters in lipid droplets. Astrocytes export most of the cholesterol they make.
The feedback mechanism regulating synthesis of cholesterol is controlled by a group of proteins that act as DNA transcription factors. Their name is sterol-regulatory element-binding proteins (SREBPs). When cholesterol is sufficient within the cell, SREBPs remain attached to the endoplasmic reticulum membrane. A low level of cholesterol causes the SREBPs to detach and move into the nucleus. There they increase message for the synthesis of the enzymes needed to make cholesterol. The level of SREBPs in the nucleus drops as cholesterol rises.
Neurons eliminate any overproduction of cholesterol by converting it to 24-hydroxycholesterol. 24-hydroxycholesterol easily crosses the blood brain barrier into capillary plasma. This enzymatic conversion occurs primarily in neurons. However, not all 24-OHC is a waste product in brain. It also activates the neuron and glial nuclear receptors LxRα and LxRβ. LxRα and LxRβ receptors increase synthesis of cholesterol transport proteins.
Cholesterol’s role at neuron synapses
Membrane cholesterol is critical for neuron signaling. A synapse is the place where neurons contact each other or contact another cell. The most common type of synapse in the brain is one where a small molecular weight chemical called a neurotransmitter is released from an axon terminal.
Neurotransmitter is stored in the axon terminal surrounded by cell membrane in structures called vesicles. The section of the axon terminal dedicated to neurotransmitter release is called the presynaptic compartment.
Neurotransmitter released from the presynaptic compartment diffuses into a gap about 20 nanometers between the presynaptic compartment and a modified section of the cell membrane across the gap named the postsynaptic density. The gap itself is called a synaptic cleft.
When the postsynaptic cell is a brain neuron, the postsynaptic density may be on a dendrite, on the neuron body or on an axon’s terminal.
For vesicles to release their neurotransmitter from the presynaptic compartment their membrane must merge with the plasma membrane bilayer. The membrane curvature needed to accomplish this depends upon its lipid composition.
Cholesterol is a component of synaptic vesicle membrane. Its role there is to support the membrane curvature necessary during the fusion process. Disturbance of cholesterol homeostasis has a negative impact on this process.
Membrane proteins called receptors that respond to the presence of neurotransmitter are embedded in the postsynaptic density. These receptors as part of their function rapidly diffuse laterally within the postsynaptic density. Receptor movement is controlled by scaffold proteins under the lipid bilayer. Efficiency of this process depends upon the presence of the correct amount of cholesterol.
In healthy normal brain, synapses that cease to function are pruned away by the glial cells. Dendrites with non-functional connections disappear. This keeps useless circuitry from slowing the high activity regions of the brain.
What has been learned about cholesterol in Alzheimer’s brain?
What role may cholesterol disfunction play in the development of Alzheimer’s disease? While a direct causal link between cholesterol and Alzheimer’s disease is unproven, much about cholesterol’s housekeeping role in the brain points to it as a possible participant in the neuron loss observed. Some investigators report a decrease in neuron membrane cholesterol in Alzheimer’s brain. Whether this reflects faulty cholesterol synthesis or of the delivery of astrocyte cholesterol to the neurons is unclear.
What is certain, however, is that people with the APOE4 allele are at risk for developing sporadic late-onset Alzheimer’s disease. About 65-80% of sporadic late-onset Alzheimer’s patients carry at least one APOE4 allele. In contrast, the prevalence of APOE4 in the general population is 15-20%. One allele of APOE4 increases risk of developing Alzheimer’s by four-fold over those carrying only the APOE3 or APOE2 allele. Two alleles of APOE4 increase the risk by ten-fold.
APOE is also produced in the liver to carry plasma cholesterol. Persons with the APOE4 allele are additionally at risk for cardiovascular disease, atherosclerosis and high LDL cholesterol in blood. Cardiovascular disease adversely disturbs brain capillaries as it does capillaries elsewhere. Yet, memory loss associated with slow development of Alzheimer’s disease over decades is currently believed to be caused by APOE4’s activity within neurons and microglia.
Three major theories are proposed to explain APOE4’s role in neuron cell death and memory loss. First, APOE4 may have a shorter functional half-life than APOE3 or APOE2. APOE3 and APOE4 differ by only one amino acid. However, that one amino acid change has a profound effect on the overall protein shape of APOE. Some suggest that the secondary structure of the APOE4 protein makes it much more susceptible to proteolysis, that its life span is shorter than that of the other isoforms and its delivery of cholesterol to and within neurons less efficient.
The second theory is that APOE4 slows the clearance from the brain of amyloid-β, a normal regulator of neuron signaling rate. Excess, uncleared amyloid-β binds to itself, changing it from a floppy single strand peptide to a diversity of multi-strand, rigid structures.
A variety of cells in brain normally clear single-stranded amyloid-β. Microglia internalizes it by pinocytosis and delivers it to lysosomes for destruction. Astrocytes and endothelial cells of the brain’s blood capillaries working together use the LRP1 receptor to move single-strand amyloid-β out into the general circulation.
Soluble amyloid-β does not appear to be incorporated into APOE packages before binding to LRP1. Rather, data indicate it binds directly to LRP1 competing with APOE for LRP1 binding sites. The competition between amyloid-β and APOE opens the possibility that APOE variant alleles may deferentially influence amyloid-β binding to LRP1.
A third theory is that APOE4 is more likely to be broken into fragments that APOE3 because of the difference in their secondary structure. There is some data that APOE4 cell fragments enter the nuclear compartment of neurons. It is proposed that these fragments may act as transcription factors regulating new protein production. A drop in cell cholesterol could be achieved if enzymes for its synthesis were affected.
Research is in progress testing each of these theories. If you are interested in finding the original papers in support of what is summarized here, check out the published reviews listed below.
Open Access Published Reviews
Rohn TT and Moore ZD (2017) Nuclear Localization of Apolipoprotein E4: A New Trick for an Old Protein. Int J Neurol Neurother. 4(2):pii: 067. doi: 10.23937/2378-3001/1410067. Epub 2017 Jul 31. PMID:29264400 click here.
Zhang J and Liu Q (2015) Cholesterol metabolism and homeostasis in the brain. Protein Cell 6(4):254-64 doi: 10.1007/s13238-014-0131-3. Epub 2015 Feb 15. PMID:25682154 click here.
Martin MG, Pfrieger F, Dotti CG (2014) Cholesterol in brain disease: sometime determinant and frequently implicated. EMBO reports 15(10):1036-1052 doi: 10.15252/embr.201439225. Epub 2014 Sep 15. PMID:25223281click here.
Verghese PB, Castellano JM, Garai K, WangY, Jiang H, Shah A, Bu G, Frieden C, Holtzman DM (2013) ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc Natl Acad Sci U S A 110(19):E1807-16. doi: 10.1073/pnas.1220484110. Epub 2013 Apr 25. PMID:23620513 click here.